The life sciences industry increasingly relies on advanced software to manage complex processes, data, and regulatory requirements. As research and development progress, the demand for specialized software tailored to laboratory workflows, data organization, and regulatory compliance grows.
This article delves into the specific software needs of the life sciences sector, exploring the functionalities, benefits, and market growth of essential software solutions that drive scientific innovation and operational excellence.
Specific Software Needs in Life Sciences
Laboratory Information Management Systems (LIMS)
Laboratory Information Management Systems (LIMS) are indispensable in life science software solutions. These systems streamline laboratory processes by handling samples, integrating data, and ensuring regulatory adherence. Major functions encompass:
- Sample management: Overseeing the lifecycle of samples from receipt to disposal, including logging details, storage locations, and chain of custody tracking.
- Data integration: LIMS connects with a variety of laboratory instruments and other life sciences data integration software platforms to facilitate seamless data exchange, reducing manual data entry and minimizing errors.
- Workflow automation: Routine tasks such as sample logging, testing procedures, and result reporting are automated, significantly enhancing lab efficiency.
- Reporting and analytics: LIMS offers powerful tools for life science data analytics software and reporting, crucial for research, quality control, and regulatory submissions.
- Compliance and security: LIMS helps to maintain data integrity and compliance with regulatory standards like GLP (Good Laboratory Practice), GMP (Good Manufacturing Practice), and 21 CFR Part 11.
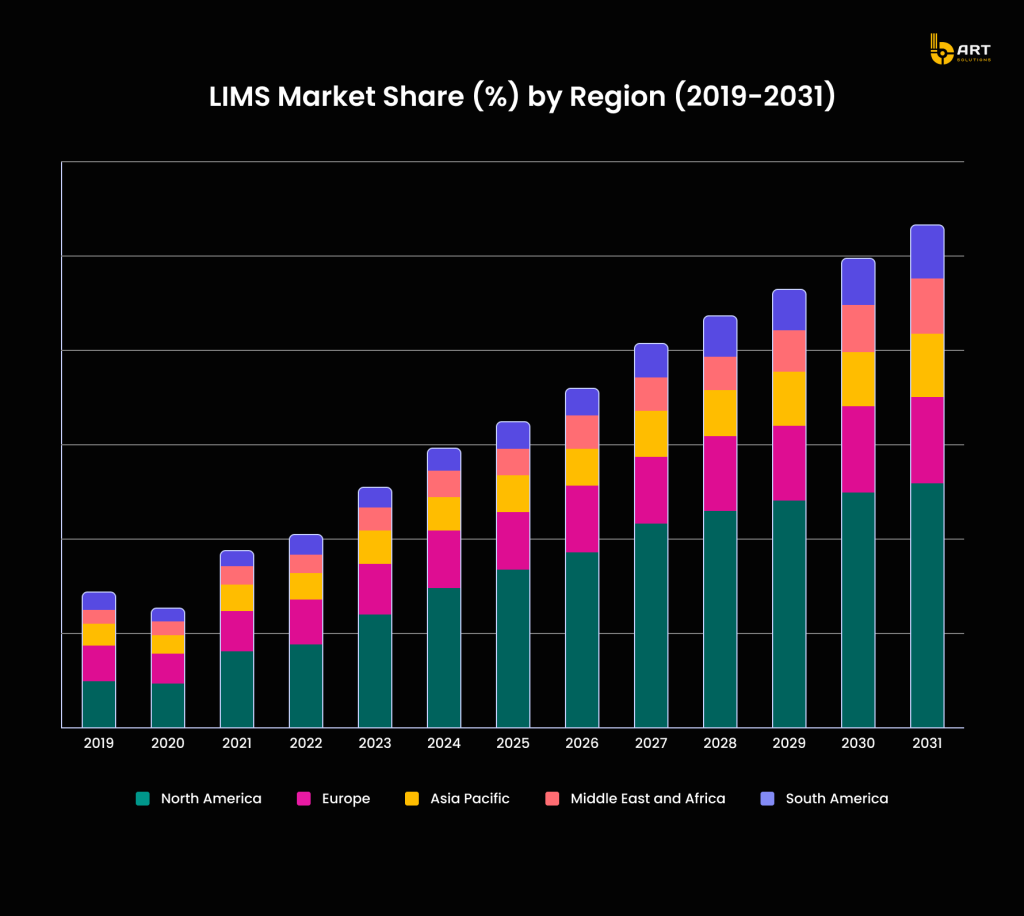
The market for life sciences software companies providing LIMS is expanding rapidly, with a projected compound annual growth rate (CAGR) of 10.6% from 2024 to 2031. The market size is anticipated to reach $15.2 billion in 2024.
Electronic Lab Notebooks (ELN)
Electronic Lab Notebooks (ELNs) represent a significant advancement in life science software applications, replacing traditional paper notebooks. Their core functionalities include:
- Data organization: ELNs provide a structured environment for recording experimental data, making data retrieval and analysis more efficient.
- Collaboration: ELN applications facilitate real-time data sharing and collaboration among researchers, even if geographically dispersed, through life science visual collaboration software.
- Searchability: Unlike paper notebooks, ELNs allow for quick and easy searching of past experiment records.
- Security: Safety measures include feature access controls, electronic signatures, and audit trails to ensure data integrity and security.
- Regulatory compliance: ELNs are helpful in complying with regulatory standards by maintaining accurate and complete records, crucial for life sciences compliance software.
The market for life sciences software vendors offering ELNs is expected to grow significantly, from $479.52 million in 2023 to $1.55 billion by 2031, with a CAGR of 13.9%. Despite this, current adoption remains low, with less than 10% of researchers using ELNs.
Clinical Trial Management Systems (CTMS)
Clinical Trial Management Systems (CTMS) are specialized software solutions for life science designed to manage the intricacies of clinical trials. Key features include:
- Study planning and design: Tools for designing and planning clinical studies, including protocol development and trial setup.
- Participant management: Tracking recruitment, enrollment, and retention of trial participants.
- Data management: Collecting, storing, and analyzing clinical trial data to ensure accuracy and compliance.
- Regulatory compliance: Ensuring that trials adhere to regulatory requirements such as Good Clinical Practice (GCP) and FDA guidelines.
- Reporting and analytics: Generating reports and performing data analysis to support decision-making and regulatory submissions.
- Financial management: Managing budgets, payments, and financial reporting for clinical trials.
The healthcare & life sciences software market for CTMS is experiencing robust growth due to the increasing complexity of clinical trials and the need for efficient management. Significant investments are being made to improve trial management processes.
Regulatory Compliance Software
Regulatory compliance software is critical for ensuring that laboratories and clinical trials adhere to various regulatory standards. Primary functionalities include:
- Document management: Efficiently managing and storing regulatory documents, leveraging document control software for life sciences.
- Audit trails: Keeping detailed records of all actions and changes for regulatory audits.
- Compliance monitoring: Continuously monitoring compliance with regulatory requirements.
- Reporting: Generating comprehensive reports to demonstrate compliance to regulatory bodies.
- Training management: Ensuring that staff receive the necessary training and certifications to meet compliance standards.
Challenges in Software Development for Life Sciences

Data Integration and Management
Diverse data sources and formats
One of the primary challenges in life sciences software development is managing and integrating diverse data sources and formats. Laboratories and research institutions often deal with a variety of data types, including experimental results, clinical trial data, and genomic information. This diversity can complicate data integration and management.
Solutions: Custom middleware, data lakes, and ETL processes
To address these challenges, life sciences software companies often employ custom middleware, data lakes, and ETL (Extract, Transform, Load) processes. Custom middleware facilitates communication between different systems, ensuring seamless data exchange. Data lakes provide centralized storage for structured and unstructured data, making it easier to manage large volumes of information. ETL processes transform data into a usable format for analysis, enhancing the functionality of life science data management software and life sciences data integration software.
Scalability and Performance
Handling large datasets and high-throughput processing
Managing large datasets and ensuring high-throughput processing are critical for life sciences software applications. As data volumes increase, maintaining system performance and scalability becomes a significant challenge.
Solutions: Cloud computing, microservices architecture
Cloud computing and microservices architecture offer effective solutions. Cloud platforms provide scalable storage and processing power, which can be adjusted based on demand. Microservices architecture allows applications to be divided into smaller, independent services, facilitating easier scaling and maintenance. This approach is particularly beneficial in life sciences software development, where flexibility and performance are crucial.
Security and Compliance
Ensuring data privacy and meeting regulatory requirements
Security and compliance are critical in the development of healthcare & life sciences software. Ensuring data privacy and meeting regulatory requirements, such as GDPR and HIPAA, is essential for protecting sensitive information.
Solutions: Custom encryption, audit trails, and access controls
Life sciences compliance software often incorporates custom encryption, audit trails, and robust access controls. Custom encryption protects data at rest and in transit, while audit trails maintain detailed records of all actions performed on the data. Access controls ensure that only authorized users can access sensitive information, enhancing the security of life science data software.
User Experience (UX)
Catering to non-technical end-users
Creating an intuitive and user-friendly interface is essential for life sciences software solutions. Non-technical users, such as researchers and clinicians, need software that is easy to navigate and use.
Solutions: Intuitive UI/UX design, role-based interfaces
To improve the user experience, life sciences software vendors focus on intuitive UI/UX design and role-based interfaces. An intuitive interface simplifies navigation and task completion for non-technical users. Role-based interfaces provide customized views and functionalities based on the user’s role, ensuring that users have access to the tools and information they need without being overwhelmed by unnecessary features. This approach is particularly beneficial in life science custom software and life science lab software.
Custom Solutions Tailored for Life Sciences

AI and Machine Learning for Predictive Analytics, Drug Discovery, Personalized Medicine
AI and Machine Learning are revolutionizing the life sciences sector by enabling advanced life science analytics software. These technologies are employed in various use cases, such as predictive analytics to forecast disease outbreaks or patient outcomes, drug discovery to identify potential new drugs faster, and personalized medicine to tailor treatments to individual patients based on their genetic makeup and other factors.
Implementation: Custom algorithms, data models
Implementing AI and machine learning in life science software solutions involves developing custom algorithms and data models. These algorithms can analyze vast amounts of data to identify patterns and make predictions. Custom data models are tailored to the specific needs of the life sciences industry, ensuring accurate and relevant insights. For instance, companies might use life sciences data software to create predictive models for patient response to treatments.
Blockchain for Secure Patient Data, Transparent Clinical Trials
Blockchain technology offers significant benefits for the life sciences sector, particularly in enhancing data security and transparency. It can be used to secure patient data, ensuring that sensitive information is stored safely and only accessible to authorized personnel. Additionally, blockchain can make clinical trials more transparent by providing an immutable record of all trial data, which helps prevent data tampering and fraud.
Implementation: Custom smart contracts, decentralized databases
The implementation of blockchain in life sciences software applications involves creating custom smart contracts and decentralized databases. Smart contracts are self-executing contracts with the terms of the agreement directly written into code. These can automate and enforce contract terms without the need for intermediaries. Decentralized databases ensure that data is not stored in a single location, reducing the risk of data breaches and enhancing security.
Internet of Things (IoT) for Real-Time Monitoring andConnected Lab Equipment
The Internet of Things (IoT) is transforming the life sciences industry by enabling real-time monitoring and connecting lab equipment. IoT devices can monitor patient vitals, environmental conditions in labs, and the status of lab equipment, providing real-time data that can improve decision-making and operational efficiency.
Implementation: Custom IoT platforms, integration with LIMS
Implementing IoT solutions in life science custom software involves developing custom IoT platforms and integrating these with Laboratory Information Management Systems (LIMS). Custom IoT platforms can collect and analyze data from various devices, while integration with LIMS ensures that all data is centralized and easily accessible. This enhances the capabilities of life science lab software by providing comprehensive monitoring and management tools.
Automation and Robotics for Data Entry, Robotic Process Automation (RPA) in Labs
Automation and robotics are increasingly being used in the life sciences sector to automate repetitive tasks and enhance efficiency. Use cases include automated data entry, where robots can input data into systems with high accuracy, and Robotic Process Automation (RPA) in labs, which can handle various tasks such as sample processing and analysis.
Implementation: Custom automation workflows, integration with existing systems
The implementation of automation and robotics in life sciences software solutions involves creating custom automation workflows and integrating these with existing systems. Custom workflows are designed to automate specific tasks and processes, while integration ensures that the automated systems work seamlessly with other software and hardware used in the lab. This approach is particularly useful for life sciences supply chain software and equipment management software life science, improving overall efficiency and accuracy.
Real-World Success Stories
LIMS for Pfizer

Problem
Pfizer, a global pharmaceutical giant, faced challenges in managing its laboratory data and maintaining regulatory compliance across its extensive operations. The company needed a robust life science software solution to streamline data management, enhance compliance, and improve overall laboratory efficiency.
Custom solution
The implemented LIMS included custom integrations with Pfizer’s existing laboratory instruments and life sciences ERP software, allowing seamless data flow and centralized management. It also featured custom workflows tailored to Pfizer’s specific laboratory processes and regulatory requirements.
Outcomes
The implementation resulted in a significant reduction in data entry errors, improved compliance with regulatory standards, and enhanced laboratory efficiency. Pfizer reported a 30% increase in productivity and a 20% reduction in operational costs due to the automated workflows and real-time data management capabilities.
LIMS for Illumina

Problem
Illumina, a leader in genetic research and DNA sequencing, struggled with data integration from various sources and maintaining the accuracy and security of sensitive genetic data. The company required a life science custom software solution to manage its complex data and support its research activities.
Custom solution
The company invested in a LIMS solution that integrated with Illumina’s genetic sequencing instruments and life sciences data management software. The custom solution included robust data protection features such as audit trails and access controls, ensuring the security and integrity of genetic data. Additionally, the LIMS offered advanced data visualization tools to help researchers analyze and interpret their data effectively.
Outcomes
Illumina experienced enhanced data accuracy and security, enabling more reliable research outcomes. The custom LIMS solution also streamlined data management processes, reducing the time required for data analysis by 40%. This improved efficiency allowed Illumina to accelerate its research and development activities, ultimately leading to faster innovation and discovery.
Blockchain Implementation for Clinical Trials by ICON plc
![]()
Problem
ICON plc, a leading Contract Research Organization (CRO), faced issues with data transparency and security in its clinical trials. Ensuring the integrity of clinical trial data and maintaining participant confidentiality were critical challenges that needed addressing.
Custom solution
ICON adopted a life sciences software application incorporating blockchain technology. The custom solution included the development of smart contracts and a decentralized database to securely record and manage clinical trial data. This blockchain-based system ensured that all data entries were immutable and transparent, providing a verifiable audit trail for regulatory compliance.
Outcomes
The blockchain implementation significantly improved data transparency and security, reducing the risk of data tampering and enhancing trust among trial participants and stakeholders. ICON reported a 25% decrease in data discrepancies and a 30% improvement in the efficiency of regulatory audits, thanks to the transparent and secure data management system.
IoT-Enabled Lab Monitoring for Roche

Problem
Roche, a global leader in diagnostics and pharmaceuticals, needed a solution to monitor its laboratory equipment in real-time and ensure optimal operating conditions. The company faced frequent equipment downtime and maintenance issues, impacting its diagnostic capabilities and overall efficiency.
Custom solution
Roche implemented a custom IoT platform integrated with its life sciences lab software. This solution included IoT sensors installed on laboratory equipment to monitor parameters such as temperature, humidity, and equipment status. The IoT platform provided real-time data to the LIMS, allowing for proactive maintenance and immediate response to any deviations.
Outcomes
Roche saw a 50% reduction in equipment downtime and a 35% improvement in maintenance efficiency. The real-time monitoring capabilities allowed for proactive issue resolution, ensuring that diagnostic operations were not disrupted. This enhanced reliability and efficiency led to more accurate and timely diagnostics for patients.
These case studies illustrate how life sciences software vendors are leveraging advanced technologies to overcome challenges and deliver robust, scalable, and user-friendly solutions for the life sciences sector.
Choosing the Right Development Partner
Selecting the right life sciences software development partner is critical for ensuring the success of your project. Here are the key criteria to consider:
Industry expertise
Your development partner should have extensive experience in the life sciences sector. This includes familiarity with the specific regulatory requirements, challenges, and workflows unique to this industry.
Technological capabilities
The right partner should possess robust technological capabilities. This includes proficiency in the latest technologies such as AI, machine learning, blockchain, and IoT, which are essential for developing advanced life science software applications. Additionally, they should be skilled in integrating these technologies with existing systems, such as LIMS and ERP, to ensure seamless data flow and operational efficiency.
Past success stories
Reviewing past success stories and case studies can provide insight into a potential partner’s ability to deliver successful projects. Look for partners who have a proven track record of implementing similar solutions in the life sciences industry.
Choosing the Right Technologies for Future Scaling and Growth
When selecting a development partner, consider their ability to implement technologies that support future scaling and growth. This involves choosing scalable solutions such as cloud-based platforms and microservices architectures that can adapt to increasing data volumes and evolving business needs. Look for partners who stay abreast of technological advancements and continuously update their solutions to incorporate new features and capabilities.
bART Solutions: Your Trusted Partner
bART Solutions is well-equipped to meet the unique needs of the life sciences sector. With a strong focus on healthcare and life sciences software development, bART Solutions offers a comprehensive suite of services that include:
- Custom software development: Tailored solutions that address specific challenges and workflows in the life sciences industry.
- Integration expertise: Seamless integration with existing systems such as LIMS, ERP, and data management platforms, ensuring smooth data flow and operational efficiency.
- Advanced technologies: Implementation of cutting-edge technologies like AI, machine learning, blockchain, and IoT to enhance research capabilities, data security, and process automation.
- Proven track record: Successful projects across the healthcare domain, demonstrates our ability to deliver impactful solutions that drive innovation and efficiency.
By partnering with bART Solutions, you can leverage our industry expertise and technological capabilities to achieve your goals and ensure the success of your life sciences projects. We are committed to providing high-quality, scalable, and future-proof software solutions tailored to meet your unique needs.
